What is theranostics?
Theranostics is a personalised form of cancer treatment that combines diagnostic imaging to identify a person’s cancer cells and targeted therapy to treat their cancer. It aims to slow the growth and spread of cancer, relieve symptoms and maintain or improve quality of life. Theranostics may be considered for people with advanced cancer, when standard treatment options are unsuitable or no longer effective and symptoms are affecting quality of life.
How does theranostics work?
The term theranostics comes from the combination of two words – ‘diagnostics’ and ‘therapeutics’. Click through the phased steps below to find out how theranostics works.
Phase 1: Diagnostics
-
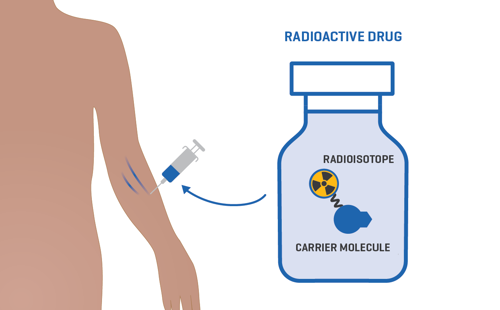
During the diagnostic stage, a radioactive drug called a radiopharmaceutical is injected into your bloodstream.
This radioactive drug is made up of a radioisotope which gives off a small amount of radiation, connected to a carrier molecule that can attach to cancer cells.
-
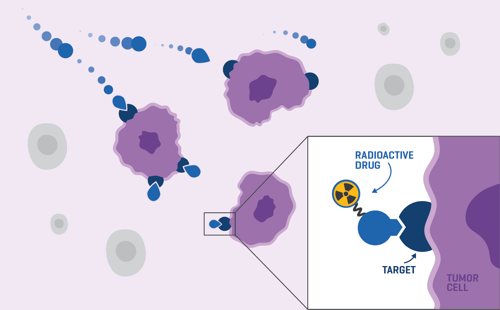
Led by the carrier molecule, the radioactive drug finds and attaches to the cancer cells in your body through a specific target on their surface.
-
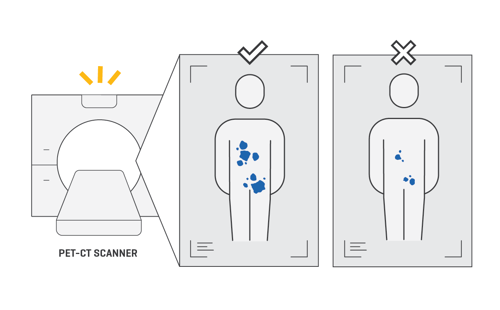
The small amount of radiation from the radioisotope then shows up as ‘hot spots’ on a PET-CT scan.
This lets doctors see where the cancer is in your body and can help them decide whether targeted therapy is a suitable treatment option for you.
Phase 2: Therapy
-
For treatment, a similar radiopharmaceutical (radioactive drug) carrying a different radioisotope is injected into your blood stream.
This radioisotope is chosen as it gives off a type of radiation that aims to injure or kill the cancer cells.

-
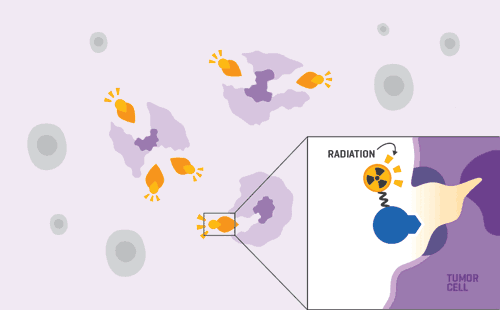
Once in your bloodstream , the radioactive drug attaches to cancer cells with the specific target.

-
The radiation released from the radioisotope injures and potentially kills the cancer cells, avoiding damage to the healthy cells around them.
Targeting this radiation as much as possible to the cancer cells reduces the potential side effects you may experience from treatment.
Is theranostics subsidised under the Medicare Benefits Scheme (MBS)?
Yes, 177Lutetium (Lutetium) prostate specific membrane antigen (PSMA) therapy is listed on the MBS for the treatment of patients with progressive or symptomatic metastatic castrate-resistant prostate cancer, where prior treatment has failed. The whole-body PSMA positron emission tomography (PET) to determine a patient’s suitability to undergo Lutetium PSMA therapy is also subsidised.
Frequently asked questions
We're here for you
If you have a question about becoming a patient at one of our centres or accessing our treatment services, we are here to help. We know that navigating a cancer diagnosis can be overwhelming, and can help you understand the process and what steps to take next.





